OphtAI is very proud to be one of the 5 AI solutions selected for a real world validation study performed by Pr. Aaron Lee and published in the prestigious ADA Diabetes Care Journal.
The OphtAI solution is one of the two solutions deemed to be usable in all ophthalmology organizations.
The results, obtained from 300,000 retinal images of 23,000 U.S. Army Veterans, confirmed OphtAI unrivaled performance, efficiency and speed in massive and automatic Diabetic Retinopathy screening. As in previous studies, it also showed the OphtAI algorithm sensitivity was superior to human readers.
January 22, 2021
The ADA Diabetes Care journal published online the results of a large validation study on automatic assessment of Diabetic Retinopathy (DR) 1. OphtAI, a joint venture between ADCIS and Evolucare, was very pleased to be part of the study as one of the 5 participants, and one of the 3 non-American companies involved in the study.
OphtAI provided two different versions of OphtAI DR, a CE marked medical device, demonstrating its recognized quality in this DR assessment study of images from a large multi ethnic, non-European population, totally independent of the acquisition equipment, and clinical protocols. OphtAI DR was shown to be the only participant in the study providing better results than an ophthalmologist, and the two OphtAI algorithms were among the three deemed clinically safe enough to be involved in the economic evaluation performed by Dr. Aaron Lee, the author of the article, and his team. The use of OphtAI DR was shown to enable reducing the screening costs by more than $15 USD per patient.
In this multicenter study more than 300,000 retinal images from over 23,000 military veterans were automatically analyzed by the OphtAI DR algorithms. Theses algorithms trained using over 800,000 images of patients screened in Paris hospitals, France, analyzed all of the images in less than 3 days (less than 1 second per image).
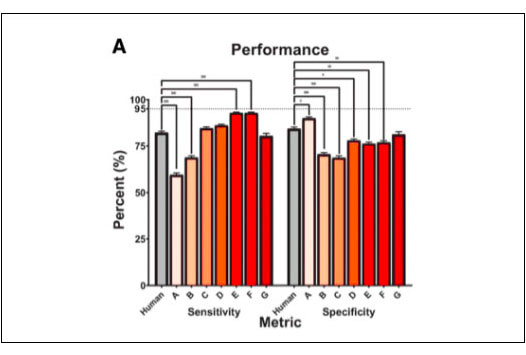
Sensitivity is the important parameter in evaluating the performance of an AI system. It measures the algorithm ability to correctly identify those patients with DR. In this study, OphtAI had the best sensitivity, and was shown to be the safest solution available on the market ready to be deployed within all screening programs worldwide. It also showed that OphtAI DR had statistically better sensitivity than human screeners.
Figure 2A*, from the published study, shows that OphtAI DR algorithms have the best sensitivity. This demonstrates that they are the safest among the seven algorithms that were provided by the participants.
Although this AI assessment study only focused on Diabetic Retinopathy, OphtAI DR was the only system in the study that can also assess other serious eye pathologies. The OphtAI DR currently on the market is CE marked for DR, diabetic macular edema, glaucoma and AMD. The second version of the OphtAI DR algorithm provided for this study had the capability of detecting more than Diabetic Retinopathy, although performance for other pathologies was not evaluated in the article. At the end of 2021, OphtAI will release a new version that will detect 37 retinal pathologies with FDA certification in progress.
American and French medical authorities advise for an annual retinal screening for diabetic patients. However, a lack of ophthalmologists in most industrial countries prevents the screening of the whole diabetic population. With OphtAI, an early screening can now be performed, with the benefit of detecting small pathologies and lesions seen in early stages of DR. In addition, the automatic screening frequency can be higher if it is performed outside hospitals and large ophthalmology organizations.
1 Multicenter, Head-to-Head, Real-World Validation Study of Seven Automated Artificial Intelligence Diabetic, Retinopathy Screening Systems”, Aaron Y Lee, Ryan T Yanagihara, Cecilia S Lee, Marian Blazes, Hoon C Jung, Yewlin E Chee, Michael D Gencarella, Harry Gee, April Y Maa, Glenn C Cockerham, Mary Lynch, Diabetes Care, 2021 Jan 5
About OphtAI
OphtAI is a joint venture specialized in Artificial Intelligence (AI) for ophthalmology, created in May 2019 by Evolucare, a leading ISE in hospital information systems for more than 30 years, and ADCIS, an SME expert in the field of image processing and analysis for 25 years. OphtAI offers a wide range of innovative solutions based on AI for semi-automatic screening of retinal pathologies. OphtAI has international ambitions and aims to become an essential reference in the Artificial Intelligence market applied to ophthalmology. Already marked CE Medical Device, the company works, in France, with the French Academy of Ophthalmology and the authorities concerned to better integrate this technology into the course of care. OphtAI is also being rolled out in many countries and targets the American market once FDA approval has been obtained.
Contact